Project:
Ball and Stick Modelling of a Jun-Fos Dimer
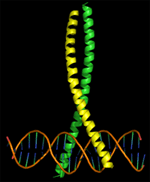 The
focus of the proposed project is to study a very interesting protein-protein
interaction called the leucine zipper (also known as a B-ZIP domain
- picture at right). Two proteins that interact in this fashion
are transcription factors Jun (Yellow) and Fos (Green). By connecting
their alpha helices they form a scissor like structure that can
bind very tightly to the DNA thus signaling to the cell to turn
genes on. In the case of the protein Jun, if it becomes malfunctional its activity can be linked to cancer. This is especially the case with a specific virus that carries with it a near duplicate of the Human Jun protein. This allows the protein to use Jun to tell the cell to start doubling - allowing the virus to double with it, but also causing cancer.
The
focus of the proposed project is to study a very interesting protein-protein
interaction called the leucine zipper (also known as a B-ZIP domain
- picture at right). Two proteins that interact in this fashion
are transcription factors Jun (Yellow) and Fos (Green). By connecting
their alpha helices they form a scissor like structure that can
bind very tightly to the DNA thus signaling to the cell to turn
genes on. In the case of the protein Jun, if it becomes malfunctional its activity can be linked to cancer. This is especially the case with a specific virus that carries with it a near duplicate of the Human Jun protein. This allows the protein to use Jun to tell the cell to start doubling - allowing the virus to double with it, but also causing cancer.
By utilizing molecular model kits, Scientific AmeriKen hopes to
construct short sections of these interacting proteins. After analyzing the structure from x-ray crystallography and the protein sequences the following regions have been selected: Human Jun (Residues 232-254) and Human Fos (Residues 161-183). 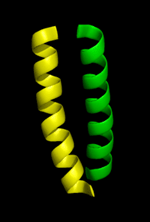
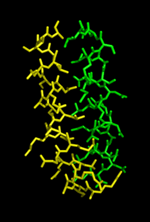 These residues have been pictured in cartoon form on the left. Once the model is constructed it will have the appearance of the structure on the right.
These residues have been pictured in cartoon form on the left. Once the model is constructed it will have the appearance of the structure on the right.
In total, this project will require the construction of 44 amino acids. The total atoms require includes 401 Hydrogens, 226 Carbons, 64 Nitrogens, 80 Oxygens. Each molecular model kit contains 28 H, 14 C, 8 O, 4 N, 12 other, therefore the total kits required is 16 at an estimated cost of $320.00.
To date one molecular model kit has been aquired. Its contents have been used to produce the following:
 Pictured on right is the largest of the Amino Acids, Tryptophan. The construction of this amino acid took nearly 95% of the kit's supplies. The total size of this amino acid was roughly 1 foot long and about 6 inches wide. Luckily there are no tryptophan amino acids in the target proteins. Click on the image to view a larger picture. Pictured on right is the largest of the Amino Acids, Tryptophan. The construction of this amino acid took nearly 95% of the kit's supplies. The total size of this amino acid was roughly 1 foot long and about 6 inches wide. Luckily there are no tryptophan amino acids in the target proteins. Click on the image to view a larger picture. |
 The amino acid pictured on the right is called Leucine. Leucines are the major contributor to the functionality of the Leucine zipper. This ability comes from hydrophobic nature (dislike of water) of leucine that allows it to seek out other leucines to form stable, protective, hydrophobic pockets, and thus leucine zippers. Click on the image to view a larger picture. The amino acid pictured on the right is called Leucine. Leucines are the major contributor to the functionality of the Leucine zipper. This ability comes from hydrophobic nature (dislike of water) of leucine that allows it to seek out other leucines to form stable, protective, hydrophobic pockets, and thus leucine zippers. Click on the image to view a larger picture. |
 In an effort to see the maximum lenght of protein that could be formed with one kit, AGSAG was formed, picture on right. AGSAG stands for amino acids Alanine-Glycine-Serine-Alanine-Glycine connected from left to right across the picture. Click on the picture to view a larger picture. In an effort to see the maximum lenght of protein that could be formed with one kit, AGSAG was formed, picture on right. AGSAG stands for amino acids Alanine-Glycine-Serine-Alanine-Glycine connected from left to right across the picture. Click on the picture to view a larger picture. |
Reference for crystal Structure:
Glover, J.N., Harrison, S.C. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature v373 pp.257-261 , 1995
Click here for the PDB file (Structure File)
Click here for a program to view the PDB file
Click here to view detailed informaton on target proteins:
Click here to view Model Kit Requirements:
If you wish to donate to the project, CLICK
HERE
Click here to return to the proposed project Home page.
Click here to discuss the Proposed Project.



