



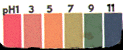 |
Pure
vinegar Sample:
Judging
from the live sampled litmus paper, the acetic
|
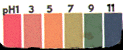 |
Chocolate/vinegar
Sample:
Judging
from the live sampled litmus paper, the Chocolate/
|
To examine
a printable version of this experiment click here
Right Click here to adjust the sound--->