Classic Combustion Reaction Experiment
 Purpose: Generally in most high school science
classes, a combustion reaction is performed where something is burned and
the effects are measured. This bi-week, Scientific AmeriKen will reproduce
one of these experiments to see whether substances gain or lose weight
when burned.
Purpose: Generally in most high school science
classes, a combustion reaction is performed where something is burned and
the effects are measured. This bi-week, Scientific AmeriKen will reproduce
one of these experiments to see whether substances gain or lose weight
when burned.

Hypothesis: In any combustion reaction, a hydrocarbon
is burned in oxygen to produce carbon dioxide and water. (see
below diagram) Because oxygen is being added and converted into the
heavier molecules of water and carbon dioxide, then it is hypothesized
that the burning of hydrocarbons results in heavier substances.
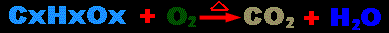 Equipment: needed for this experiment is a scale,
some kind of hydrocarbons (used in this experiment were saltine crackers),
matches, 2 plates, pen and paper.
Equipment: needed for this experiment is a scale,
some kind of hydrocarbons (used in this experiment were saltine crackers),
matches, 2 plates, pen and paper.

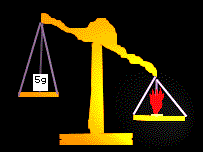
Procedure: The first step is to weigh the crackers
and the plates. Record their weights. Then Burn the cracker and place the
plate above the burning cracker to catch the water vapor and place a place
below the crackers to catch the any excess substances. Record the final
weights and compare.

Results:
| Objects Massed |
Weights
|
| Two plates & unburned cracker |
14.31 ounces
|
| Two plates & burned cracker |
14.26 ounces
|
| Net gain or loss |
.05 ounces
|

Conclusion: Based on the results it would seem
that substances lose weight when they are burned. These results contradict
the hypothesis, however, it is noted that during testing some of the smoke
escaped from the plate, which may account for the loss of weight. Further
experimenting should be done on this, however, seeing as it is a usual
high school experiment, it was probably not even designed to work right
in the first place.  Return
to Scientific AmeriKen
Return
to Scientific AmeriKen


![]()