 |
 |
| |
Limb-bud and heart, or LBH for short, is a newly discovered and relatively small protein found in most mammals, including humans. While functioning normally, it is believed to signal for the creation of limbs and the heart at the right times during embryo development. However, recent evidence suggests abnormally functioning LBH may have a role in certain types of cancer, including breast cancer. Understanding how a protein, such as LBH, "behaves" is important to developing drugs to control it. In this publication, the authors utilize a plethora of biochemical and biophysical techniques to learn more about LBH. Scientific AmeriKen assisted and will share two techniques in this review.
|
|
 |
| |
When determining the "behavior" of proteins some of the details scientist look for are whether the protein oligimerizes (i.e. exists by itself or combines with copies of itself to form dimers, trimers or more) or whether the molecular shape of the protein is rigid (protein folds tightly together) or not. LBH is believed to be a transcriptional regulator - a protein that interacts with transcription factor proteins, proteins that make direct contact with the DNA, and either enhance or block their activity. Such proteins have been known to oligimerize to form dimers and/or be poorly folded. However, preliminary work by the authors of this paper suggest that LBH may also form a dimer and Scientific AmeriKen will work under the hypothesis that it is.
|
|
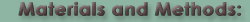 |
| |
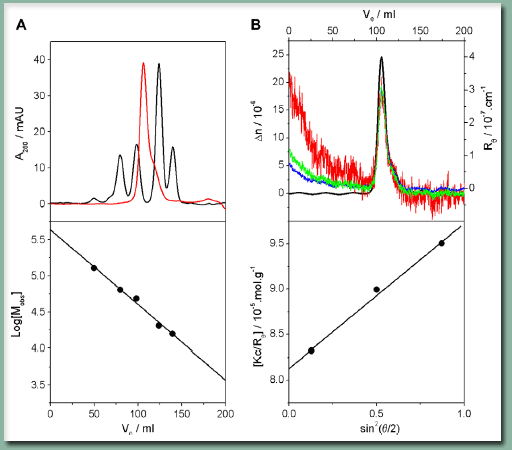
The preliminary work of the authors utilized a technique called Size Exclusion Chromatography (SEC), which Scientific AmeriKen will also use to reproduce their results. SEC, figure A on right, is a technique where a protein sample is pushed through a column under high pressure. The column is composed of fine beads that come together to form a gel matrix. As molecules, such as proteins, enter the matrix their ability to stay in it without being pushed out depends on their size - thus larger molecules leave the column quicker than smaller allowing for separation by size. By running proteins of a known molecular mass (called standards), a researcher can estimate the molecular mass of an unknown protein such as LBH. The one downside of this technique, however, is the shape of the protein also matters. The standard proteins are tightly folded and spherical in shape. Compared to these ball like proteins, an unfolded protein will fall out of the column and give a molecular mass that is larger than its actual molecular mass.
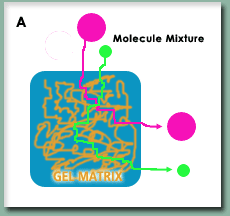
To address this possibility, Scientific AmeriKen will also utilize Analytical Light Scattering (ALS) to study the LBH protein. In ALS, figure B on right, a laser beam strikes the protein sample and the scattering, or reflection off the protein, of that light is recorded at three different angles. The amount of scattering that results is dependent on the molecular mass of the proteins - thus this technique provides the molecular mass independent of shape. With these two techniques ready to go, Scientific AmeriKen acquired purified samples of LBH and began the work to understand the behavior of this protein. |
|
|
 |
| |
Panel A of the figure on the right shows the SEC results of LBH. The black line on top represents 4 standard proteins used to make the calibration line seen on the bottom. The red line represents LBH, which in comparison to the standard proteins, suggests LBH has an apparent molecular mass of 33,000 daltons. By counting the molecular mass of all the atoms in LBH, the theoretical molecular mass is determined to be 12,300 daltons. Thus, as determined by SEC, The apparent size of LBH is 3 times that of its theoretical suggesting LBH may be a trimer.
Panel B of the figure on the right shows the ALS results of LBH. Each colored line in the top represents the scattering detected from one of the angles. The black line represents a measure of the concentration. The bottom shows a sample calculation to determine the molecular mass where each dot represents one of the angles at a specific concentration. These calculations are done several thousand times and averaged out. The ALS analysis gives a molecular mass of 11,800 daltons which is within experimental error to the theoretical value of 12,300 and suggest LBH is a monomer (doesn't work with copies of itself).
|
 |
|
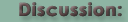 |
| |
The light scattering data agrees very closely to the theoretical molecular mass of LBH, thus suggesting this protein acts as a monomer. SEC results at first suggest the protein may behave as a trimer, or 3 copies bound to each other, however, in light of the ALS data, a better explanation is that LBH has very little structure and thus leaves the column quicker compared to the tightly folded protein standards. This would cause it to have a higher apparently molecular mass. Thus, the conflicting data arising from SEC and ALS leads to one conclusion - that LBH is a monomer that does not have structure. It is believed that having little structure increases the ability of proteins to find binding partners as the flexibility increases the range of the search. Another aspect to consider is typically proteins that are unstructured can become structured when binding to their targets. In summary, these data help to understand the "behavior" of LBH.
|
|
 |
| |
In summary, the two experiments of Scientific AmeriKen are only a part of the exhaustive analysis of the behavior of LBH. The authors utilized other techniques such as circular dichroism (examining the effect the protein has on polarized light), Nuclear Magnetic Resonance (examining the effect of magnetism on the protein sample), Steady state fluorescence (examining the ability of the protein to absorb and emit light) and computer prediction techniques to support the notion that this protein does not have a rigid structure. Such disorder may allow LBH flexibility to interact with various signaling proteins and this study provides useful insight to the design of drugs that may target this protein.
|
|
|
 |
|
